Australia | 医疗器械
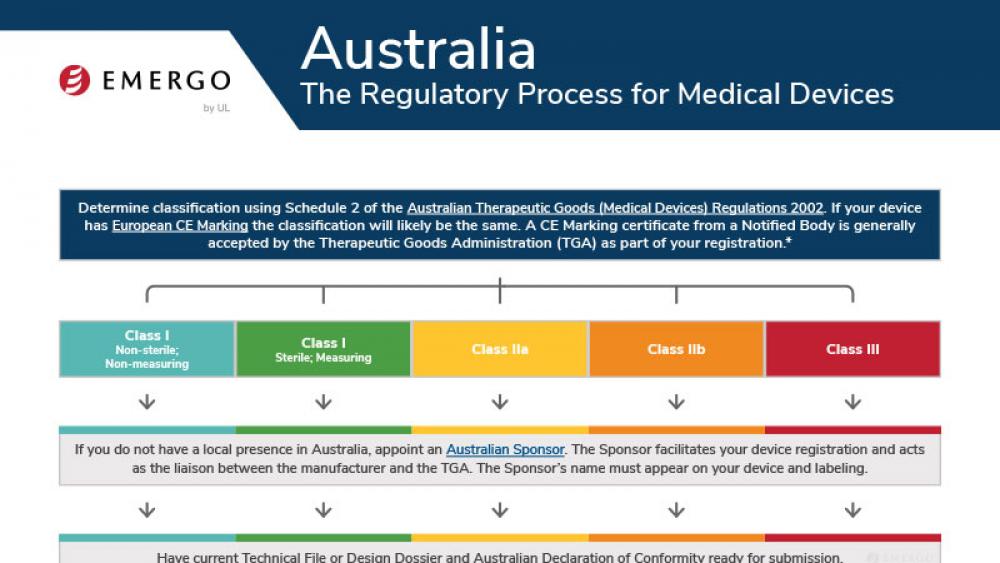
澳大利亚TGA认证和医疗器械注册审批流程
This chart illustrates the steps in the Australia TGA medical device approval process and includes a timeline of expected approval.
2 pages
2013年 8月 29日
Brazil
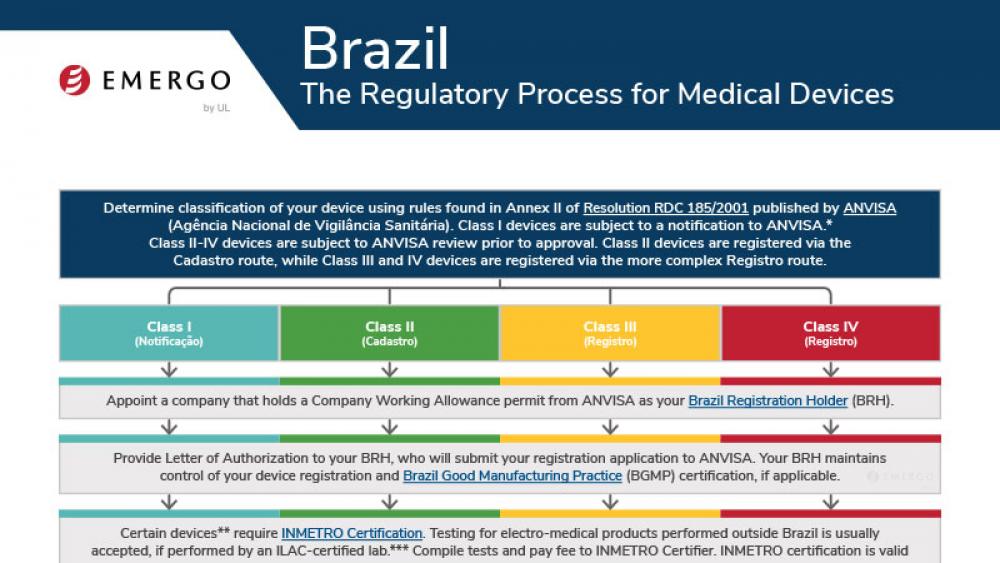
巴西ANVISA医疗器械和IVD器械认证合规监管审批流程
This chart illustrates the steps in the Brazil ANVISA medical device approval process and includes a timeline of expected approval.
2 pages
2016年 7月 21日
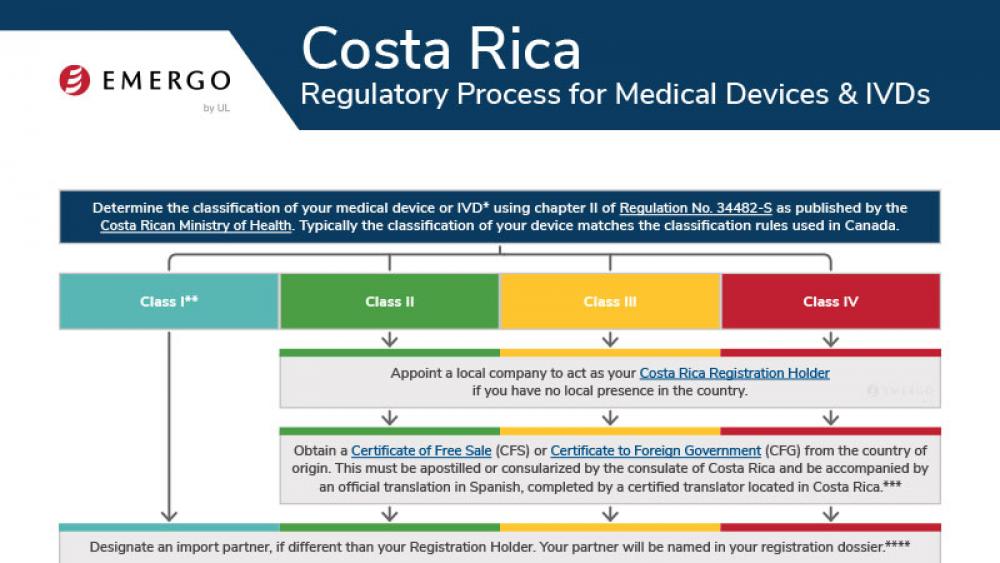
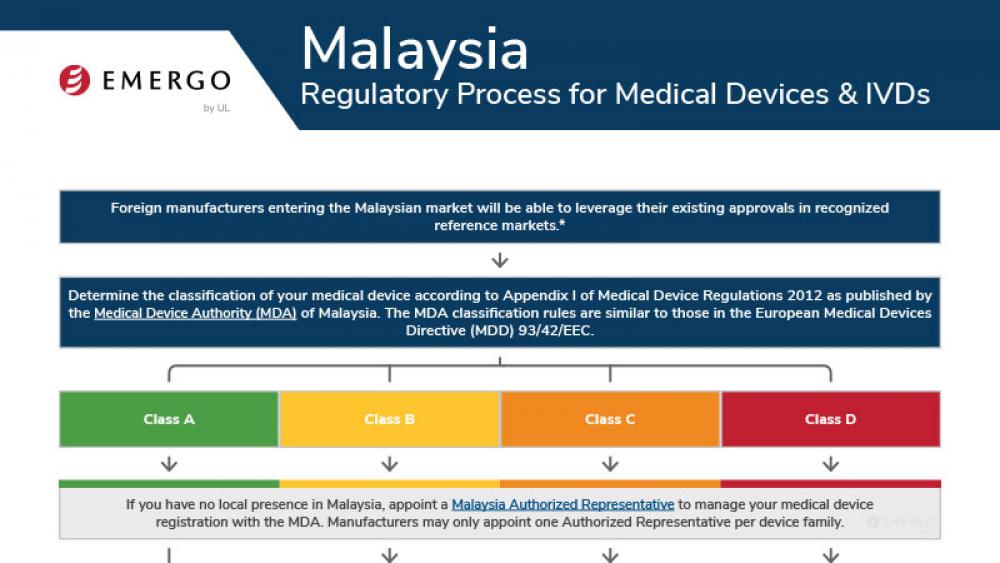
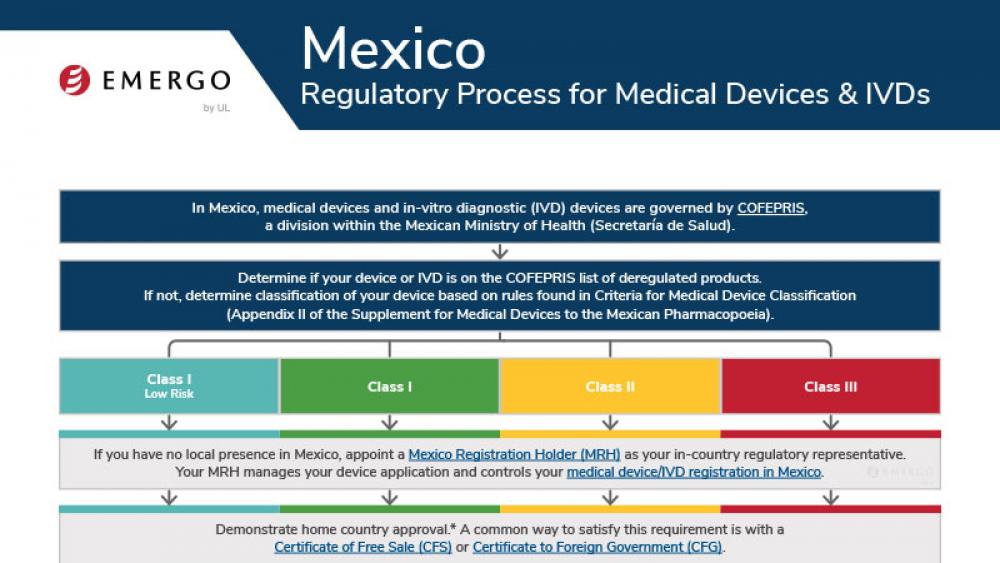
Costa Rica | 医疗器械
哥伦比亚医疗器械和IVD器械INVIMA认证合规审批流程
Medical device manufacturers who want to enter the Costa Rican market need to obtain approval from the Costa Rican Ministry of Health. There are a few pathways to approval, and documentation requirements are different depending on your device's class.
2 pages
2015年 6月 3日
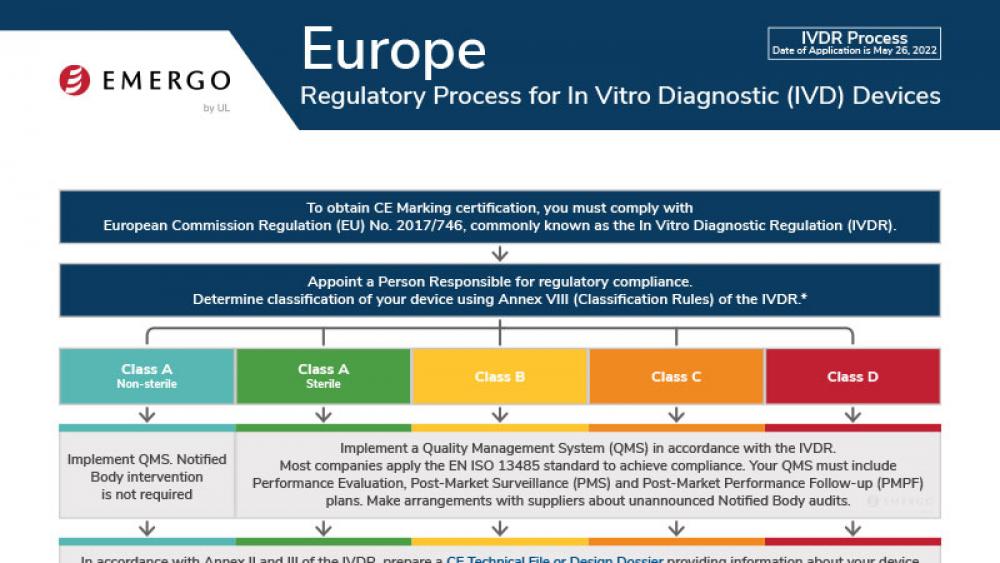
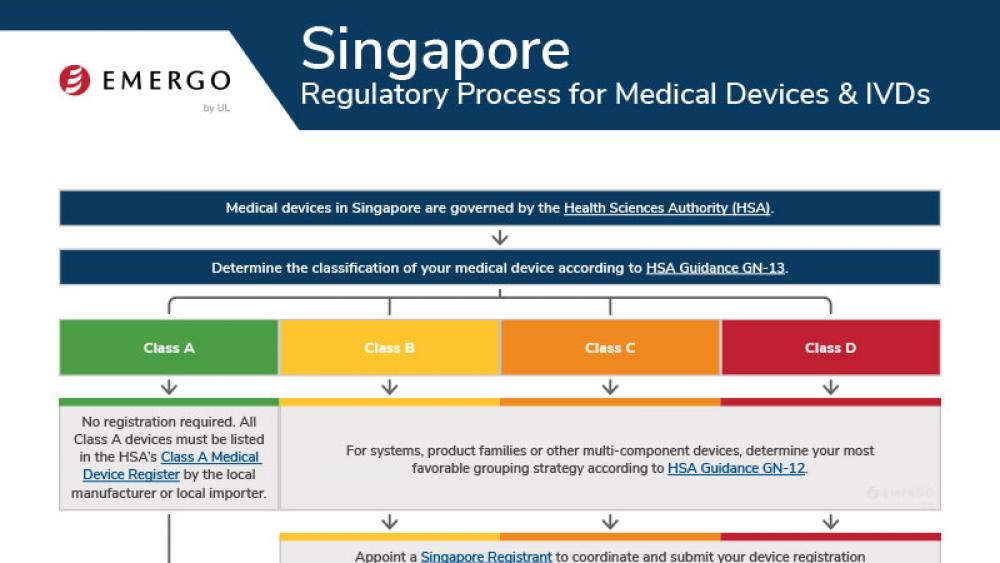
Europe | 医疗器械
欧盟MDR下的PMS和PSUR要求
这份白皮书介绍了MDR针对PMS和PSUR义务的新要求以及在全球QMS中实施这些要求所产生的风险。
9 pages
2019年 7月 23日
Europe | 医疗器械
英国医疗器械和IVD器械注册及UKCA认证合规审批流程
此流程图阐明了英国每个医疗器械分类MHRA注册认证审批流程,可在法规事务管理平台(RAMS)下载。
2023年 5月 18日
Europe | 医疗器械
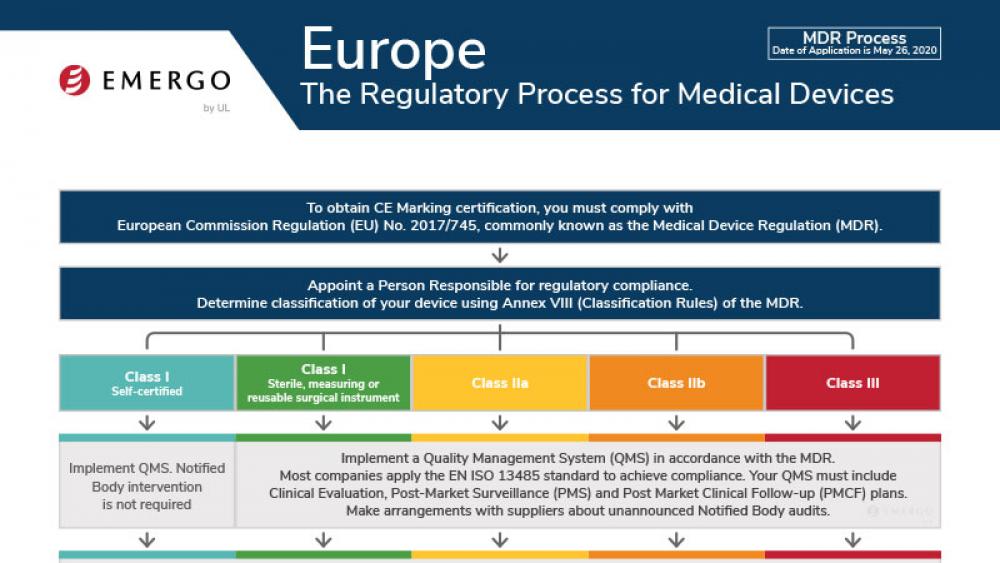
欧盟医疗器械法规(MDR)CE认证标志合规流程
This chart illustrates steps to CE Marking in Europe's Medical Devices Regulation (MDR 2017/745) approval process.
2 pages
2017年 8月 23日
Europe | 采购/供应链
欧盟IVDR准备情况评估检查清单 | Emergo by UL
这份简短的检查清单将帮助您确定如何让您的体外诊断医疗器械公司符合欧盟IVDR。
2 pages
2021年 3月 11日
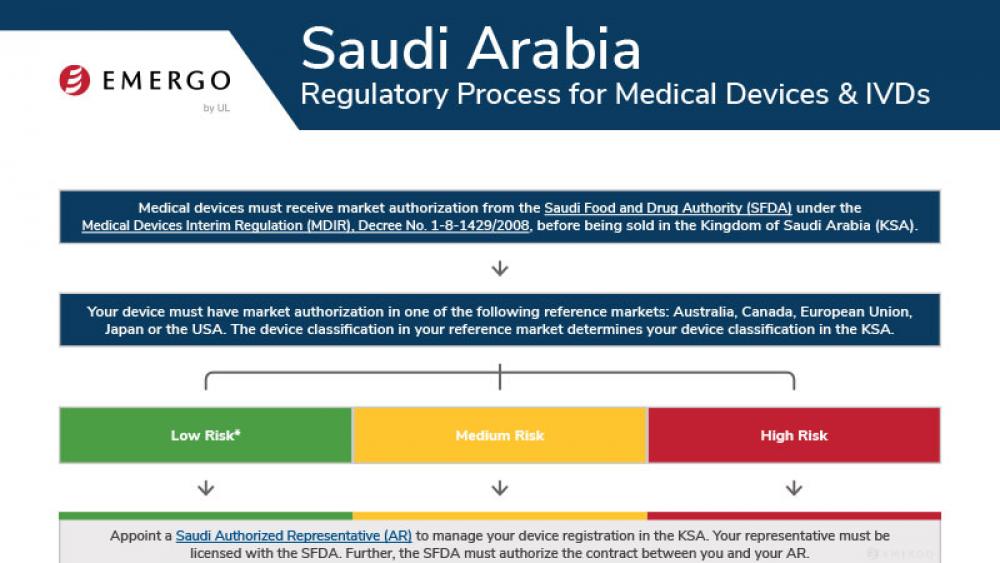
Saudi Arabia | 医疗器械
沙特医疗器械注册和SFDA认证合规审批流程
此流程图阐明了沙特阿拉伯每个医疗器械分类的注册和SFDA认证审批流程,可在法规事务管理平台(RAMS)下载。