Introduction to Saudi Arabia's Medical Device Approval Process
Saudi Arabia is one of the world's top 20 global economies. It has close to 29 million people, and about 95% of its market is supplied by imports. Interested in entering this prime market for medical devices? This short video provides an overview of how Saudi Arabia regulates medical devices. Topics covered include the Saudi Food and Drug Authority (SFDA), leveraging your device approval from a reference market (Australia, Canada, Europe, Japan, and/or the USA), requirements for quality systems from your reference market, Local Authorized Representative in Saudi Arabia, Medical Device Market Application (MDMA), and more.
Related items:
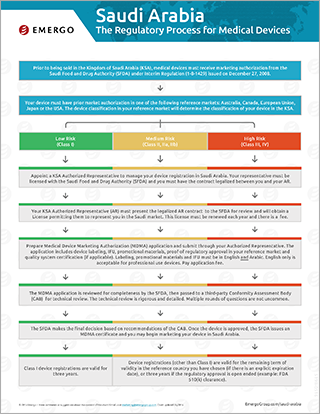 Regulatory Chart about the process to approval in Saudi Arabia
Regulatory Chart about the process to approval in Saudi Arabia
 Saudi Arabia Country Report
Saudi Arabia Country Report
Free white paper provides an overview of the medical device regulatory system in the Kingdom of Saudi Arabia.
有任何问题?向我们的专家获取相关信息
联系我们相关
-
美国FDA eSTAR电子递交模板与资源要求解析
自2018年起,美国食品和药物管理局(U.S. Food and Drug Administration,FDA)开始试行以电子化方式来帮助行业提供完整的510(k)上市前通知。2022年,为推进以电子格式提供510(k)递交文件的过渡,美国FDA正式发布关于使用电子递交模板和资源的最终指导文
阅读更多 -
欧盟医疗器械法规MDR 全新解读 – 共瞻2022医疗器械合规新要点
自新欧盟医疗器械法规MDR (Medical Device Regulation 2017/745) 申请日期起,欧盟医疗器械监管当局就发布了一系列新文件及相关支持服务,旨在帮助医疗器械制造商适应新法规变更后的法规环境。 对此,作为在全球范围内经验丰富的医疗器械及IVD器械市场合
阅读更多