FDA Report: More QSR Inspections at Foreign Sites in 2014
有任何问题?向我们的专家获取相关信息
联系我们2015年 11月 9日
The US Food and Drug Administration increased its number of quality system inspections at foreign medical device manufacturing sites in 2014, expanding on its years-long effort to monitor FDA Good Manufacturing Practice compliance at sites based outside the US.
According to the report, the FDA performed more 21 CFR Part 820 quality management system inspections in 2014 than in previous years, and also increased its inspections of US market registrants based in other countries. A higher percentage of observations and warning letters were issued to foreign registrants than to US-based firms.
More foreign firms undergoing inspection
The FDA has made a priority of inspecting more foreign device manufacturers and manufacturing sites in recent years as these firms’ inventories have increased, the report states. In 2014, the agency conducted quality system inspections at 594 foreign sites compared to 460 in 2013. Inspections of US-based manufacturers decreased over the same period, from 1741 in 2013 to 1619 in 2014. This trend shows that the FDA is steadily devoting more if its resources to overseas inspections at the small but not insubstantial expense of the agency’s domestic inspection regime.
Since 2011, the agency has steadily increased its QSR inspections of foreign sites, while at the same time slightly reducing the number of inspections carried out in the US. In 2011, about 85% of all FDA QSR inspections occurred in the US versus 15% abroad; in 2014, the percentage of quality system inspections in the US fell to about 73%, with 27% of inspections conducted at foreign sites.
Number of US versus Foreign FDA QSR Inspections 2011-2014
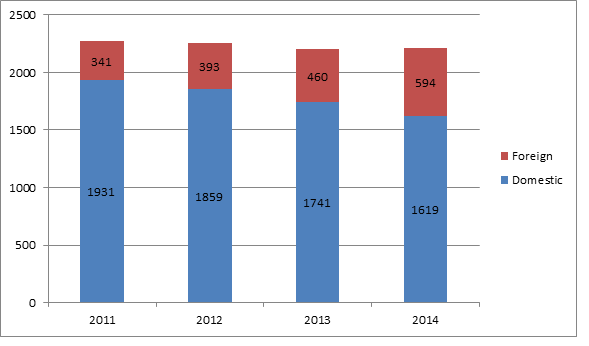
Source: US FDA
Most foreign quality system inspections occurred in China (190), followed by Germany (72), Japan (37) and Taiwan (29). Of the top 10 foreign inspection locations, a strong majority of sites (279) were in Asia, reflecting the region’s export-oriented outlook.
Higher percentage of observations for foreign registrants
Since the FDA increased its foreign inspections in 2014, it is perhaps not surprising that inspectors reported a higher percentage of observations—particularly official actions—among foreign sites with little to no experience undergoing such inspections.
|
US-based Inspections |
Foreign Inspections |
||
|
Decision |
Percentage |
Decision |
Percentage |
|
No action indicated |
52% |
No action indicated |
42% |
|
Voluntary action indicated |
40% |
Voluntary action indicated |
43% |
|
Official action indicated |
8% |
Official action indicated |
15% |
Source: US FDA
Overall, QMS inspection outcomes in which no action was indicated increased slightly from 52% in 2013 to 54% in 2014; since 2008, no action indicated results have held steady at just above 50%, the report shows. Voluntary actions indicated also decreased slightly to 38% of inspections in 2014, from 41% in 2012 and 2013. Official actions indicated, however, rose slightly to nine percent in 2014 from eight percent the previous year. Again, the small bust steady rise in official action percentages can likely be attributed to more inspections of foreign sites unfamiliar with QSR compliance requirements.
Types of observations: P&PC, CAPA still most frequent
According to the report, inspectional observations of both US and foreign device manufacturing sites totaled 3,740, the bulk of which pertained to Production and Process Controls (P&PC; 32% of all observations) and Corrective and Preventative Actions (CAPA; 31%).
These findings illustrate again that regardless of where manufacturers produce their FDA-registered devices, P&PC and CAPA issues warrant careful and consistent attention in order to meet QSR compliance requirements.
In terms of Form 483 observations, 57% of foreign sites inspected were issued Form 483s, while 47% of US-based sites received Form 483s. As many of these foreign sites likely underwent QSR inspections for the first time, it makes sense that these sites would rack up more Form 483s than stateside manufacturers more experienced in dealing with inspectors.
A considerably higher number of US-based rather than foreign manufacturing sites received warning letters with quality system citations, however. Although the total number of warning letters issued in 2014 fell to 121 from 144 in 2013 and 144 in 2012, the number of sites in the US receiving such letters rose to 76 from 70 the previous year. Only 45 foreign sites received warning letters in 2014, on the other hand, compared to 74 in 2013.
As was the case with quality system and Form 183 observations, most warning letters issued in 2014 pertained to CAPA (90% of letters) and P&PC (85%) subsystems.
Additional information on this issue can be found in Emergo’s whitepaper on the FDA QSR inspection process.
作者
- Stewart Eisenhart