FDA Data Shows Increase in Warning Letters to Medical Device Firms Since 2005
有任何问题?向我们的专家获取相关信息
联系我们2013年 5月 8日
US Food and Drug Administration medical device quality system inspectors have issued warning letters to manufacturers at an increasing rate since 2005, FDA inspection records show.
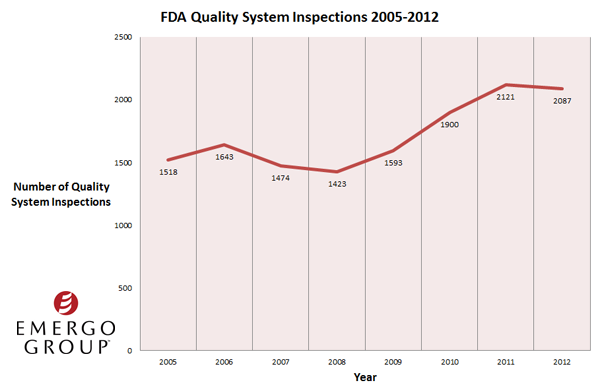
The steady increase in Form 483 observations and warning letter citations coincides with higher rates of FDA Quality System Regulations compliance inspections since 2008. (FDA officials conducted 1423 quality system inspections at medical device manufacturers in 2008, compared to 2121 in 2011 and 2087 in 2012.) Clearly the more quality system inspections US regulators conduct, the more noncompliance issues they observe and cite.
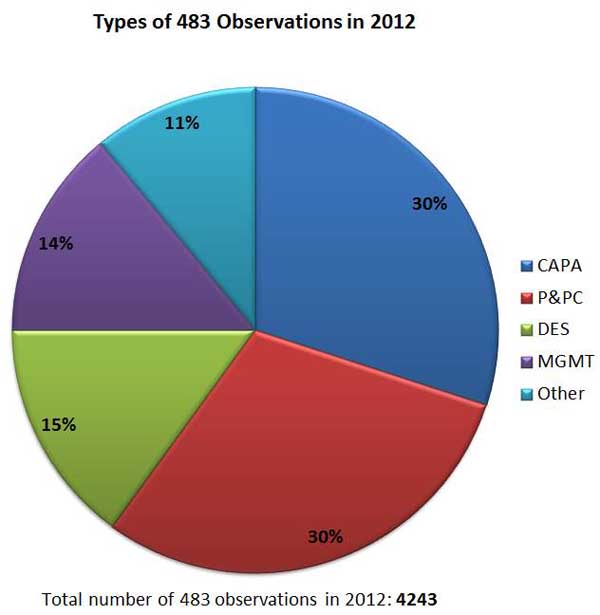
But which types of noncompliance issues are inspectors most commonly finding--and which quality system components should medical device manufacturers therefore pay more attention to going forward to avoid Form 483 warning letters or citations? Based on 2012 data, firms should pay closer attention to Corrective and Preventative Action (CAPA) and Production and Process Controls (P&PC). CAPA and P&PC issues each accounted for 30% of 483 observations last year, well ahead of Management, Design and Document Controls.

Quality system observations resulting in citations have also dramatically increased since the FDA began its more aggressive inspection schedule. Since 2009, the number of warning letters has climbed steadily, with the single biggest increase between 2011 and 2012. Has the increase in these citations resulted from a recent slump in quality system compliance among medical device manufacturers? Probably not--more likely, higher frequency of inspections has identified issues that may have been missed by less frequent and vigorous inspections prior to 2009.
Takeaways: The FDA 483 warning letter data supports medical device industry claims that quality system citations have increased over the last four years. Medical device firms should expect the trend of stepped-up FDA inspections to continue through the Obama administration's second term. However, the data also provides a clear indication of which QSR quality system components most commonly draw scrutiny from inspectors; medical device manufacturers active in the US market now know to pay particular attention to their CAPA and P&PC procedures to ensure compliance with 21 CFR Part 820.
作者
- Stewart Eisenhart