In Depth: US FDA Medical Device Registration and Compliance
Information current as of January 2017.
The US market represents more than 40% of the global market for medical devices. Yet for many manufacturers, the process of obtaining clearance from the US FDA can be daunting. In this seven part slidecast we will demystify the FDA device clearance process, breaking it down into understandable steps and explaining each one. Once you begin the series you may skip to the next section at any time.
Here is what we will cover:
- An overview of the US market and regulatory framework [runtime = 3:58]
- The steps involved in classifying your medical device [runtime = 3:56]
- Quality Management System requirements [runtime = 3:25]
- Product testing and clinical data requirements [runtime = 3:08]
- Registration dossier preparation [runtime = 5:12]
- In-country representation for companies with no US office [runtime = 2:08]
- Device listing on the FDA website and maintenance [runtime = 2:39]
We recommend:
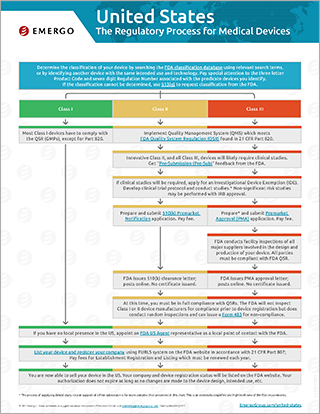 Download the free chart: USA Regulatory Approval Process for Medical Devices
Download the free chart: USA Regulatory Approval Process for Medical Devices
All companies planning to sell a medical device in the United States need to register their product with the US FDA. This chart illustrates the FDA approval process in the USA, and is available for download in PDF format. Download now.
有任何问题?向我们的专家获取相关信息
联系我们相关
-
美国FDA eSTAR电子递交模板与资源要求解析
自2018年起,美国食品和药物管理局(U.S. Food and Drug Administration,FDA)开始试行以电子化方式来帮助行业提供完整的510(k)上市前通知。2022年,为推进以电子格式提供510(k)递交文件的过渡,美国FDA正式发布关于使用电子递交模板和资源的最终指导文
阅读更多 -
Eudamed在MDR和IVDR下的作用
欧盟医疗器械数据库(Eudamed)是欧盟新医疗器械和IVD法规的重要组成部分。 Eudamed是一个数据库,用于根据医疗器械法规(MDR 2017/745)和体外诊断医疗器械法规(IVDR 2017/746)来监控器械的安全性和性能。 医疗器械业界渴望了解他们需要如何与Eudamed互动,以
阅读更多