In Depth: US FDA Medical Device Registration and Compliance
Information current as of January 2017.
The US market represents more than 40% of the global market for medical devices. Yet for many manufacturers, the process of obtaining clearance from the US FDA can be daunting. In this seven part slidecast we will demystify the FDA device clearance process, breaking it down into understandable steps and explaining each one. Once you begin the series you may skip to the next section at any time.
Here is what we will cover:
- An overview of the US market and regulatory framework [runtime = 3:58]
- The steps involved in classifying your medical device [runtime = 3:56]
- Quality Management System requirements [runtime = 3:25]
- Product testing and clinical data requirements [runtime = 3:08]
- Registration dossier preparation [runtime = 5:12]
- In-country representation for companies with no US office [runtime = 2:08]
- Device listing on the FDA website and maintenance [runtime = 2:39]
We recommend:
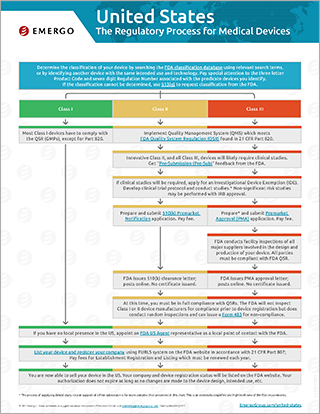 Download the free chart: USA Regulatory Approval Process for Medical Devices
Download the free chart: USA Regulatory Approval Process for Medical Devices
All companies planning to sell a medical device in the United States need to register their product with the US FDA. This chart illustrates the FDA approval process in the USA, and is available for download in PDF format. Download now.
有任何问题?向我们的专家获取相关信息
联系我们相关
-
韩国医疗器械法规框架及注册路径解读
我们的专家将详细解读2023年韩国食品药品安全部(MFDS)指导文件草案更新下韩国医疗器械注册监管要求、分类标准、韩国良好生产规范(KGMP)等相关要求,并将依据多年韩国本地注册经验提供注册案例分享。此外,您还将了解到制造商在面对MFDS监管时需要关注的核心要点,相关研讨会内容包括:
阅读更多 -
中国国家食品药品监督管理局(CFDA)医疗器械规定
以下列出中国国家食品药品监督管理局(CFDA)对於医疗器械公司会造成重大影响的文件,不过所提供的文件都只限於中文版。
阅读更多