Singapore HSA Revises Medical Device Change Notification System
有任何问题?向我们的专家获取相关信息
联系我们2015年 12月 1日
The Health Sciences Authority (HSA), Singapore’s medical device market regulator, is introducing revisions to its change notification system for medical devices effective December 1, 2015.
 New guidance from the HSA lays out several updates that the regulator claims will make the change notification process more efficient and streamlined for registered devices. Change notification entails manufacturers informing the HSA of any changes or modifications to their devices in a timely manner in order to continue legally selling in Singapore.
New guidance from the HSA lays out several updates that the regulator claims will make the change notification process more efficient and streamlined for registered devices. Change notification entails manufacturers informing the HSA of any changes or modifications to their devices in a timely manner in order to continue legally selling in Singapore.
Key change notification process revisions include:
- Adding a device with a different proprietary name to a registered device listing will not qualify for change notification, and instead require a new premarket registration with HSA.
- Adding new models to a device listing will now fall under the Review Change category of the HSA change management process.
- Following HSA approval of a change notification, a manufacturer may market both its original registered device as well as its changed medical device in Singapore only if both versions of its product conform to Essential Requirements for Safety and Performance.
The new guidance also provides a table more clearly explaining which medical device classes qualify for which of four types of change notifications (Technical Changes, Review Changes, Administrative Changes and/or Notifications):
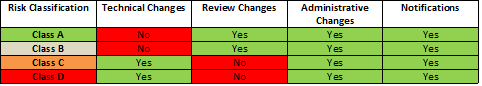
Affected Singapore registrants should familiarize themselves with the new guidance for a full list of revisions, and to determine whether and how any updates to the HSA change notification system will impact their operations.
Additional information can be found in our whitepaper and video overview on Singapore’s medical device registration process.