FDA Report: 510(k) Applicants More Responsive to Additional Information Requests
有任何问题?向我们的专家获取相关信息
联系我们2015年 11月 19日
Recent data from the US Food and Drug Administration shows that additional information requests (AI) for 510(k) medical device premarket notification applications remain high, but also that the frequency of AI requests has fallen slightly since 2010.
Percentages of 510(k) submissions that drew AI requests during their first-round reviews reached 70% in 2014, and are at 69% so far for 2015, according to a new report on the FDA’s medical device performance goals through September 30, 2015. Such requests during first-round 510(k) reviews have increased steeply and steadily between 2002 (36% of applications) and 2011, when 77% of applications warranted first-round AI requests.
Up until 2012, percentages of 510(k)s that received second-round AIs followed a similar trajectory—but then fell significantly, as illustrated in the chart below.
First- Versus Second-Round AI Requests for 510(k) Applications
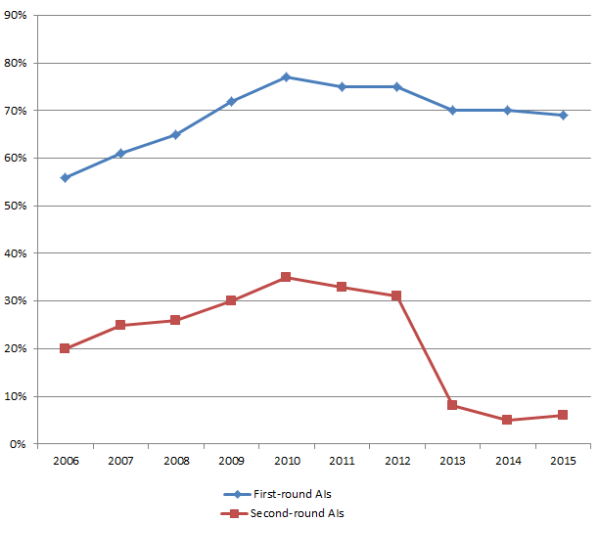
Source: US FDA
Behind the numbers
Although the MDUFA report itself does not provide analysis to explain what has driven increasing percentages of AI requests, these numbers do fall in line with the FDA’s gradually more rigorous approach to 510(k) application reviews.
During the same time period when numbers of first-round 510(k) AI requests peaked, medical device industry groups were lodging more sustained complaints about how burdensome the FDA’s registration process had become, and new management at the agency under director Margaret Hamburg had begun tightening device review processes.
The fact that AI requests began leveling off over the past few years—and fell dramatically for second-round 510(k) reviews—suggests that some 510(k) applicants have become more aware of what FDA reviewers require, but more strongly shows that applicants are doing a much better job responding to first-round AI requests so that their submissions do not garner additional AIs during second-round reviews. More explicit requests from FDA reviewers as well as more thorough responses from manufacturers may both explain this trend. So, while about 70% of 510(k) applications still receive first-round AI requests as of September 2015, more than 90% of those applications are able to meet FDA requirements upon their second round of review.
RTA policy having an effect?
Another factor to consider regarding declining first-round AI numbers is the FDA's relatively new Refuse to Accept (RTA) policy whereby premarket submissions are screened for completeness before any substantive review begins. Submissions deemed incomplete are sent back to applicants for corrections and then resubmitted. Introduced in late 2012 and enforced in 2013, could the RTA policy be affecting AI requests?
"I think that some issues that previously were raised by the FDA as AIs are now being brought to the company earlier, during the RTA process," says Audrey Swearingen, Director of Regulatory Affairs at Emergo. "So, that could result in more complete submissions by the time the FDA accepts 510(k) applications for substantive review."
Reasons for so many first-round AI requests
In a 2011 FDA report on 510(k) review timeframes analyzed by Emergo, the agency identified improper device descriptions as the biggest single reason for issuing AI requests upon first-round review of 510(k) submissions. Other issues prompting AI requests included incomplete or questionable indications for use information and failure to follow current guidance.
Although 510(k) applicants may still have trouble addressing these issues in their initial premarket submissions, more of them are now able to satisfy the FDA’s first-round AI requests so that their reviews can proceed.
作者
- Stewart Eisenhart