Analysis of 24,000+ FDA 510(k) records reveals trends
有任何问题?向我们的专家获取相关信息
联系我们2014年 2月 14日
Clients often ask us how long it will take for their medical device 510(k) submission to clear. The short answer is: it depends. But that answer doesn’t really satisfy most people. A few years ago we decided to take a more scientific approach to the issue and started examining FDA data to see how long it really takes a 510(k) to be cleared by the FDA. This year, we updated our analysis of all medical devices cleared via the 510(k) process between 2006 and the end of 2013. You can read our full analysis of FDA 510(k) review times but here are some key takeaways:
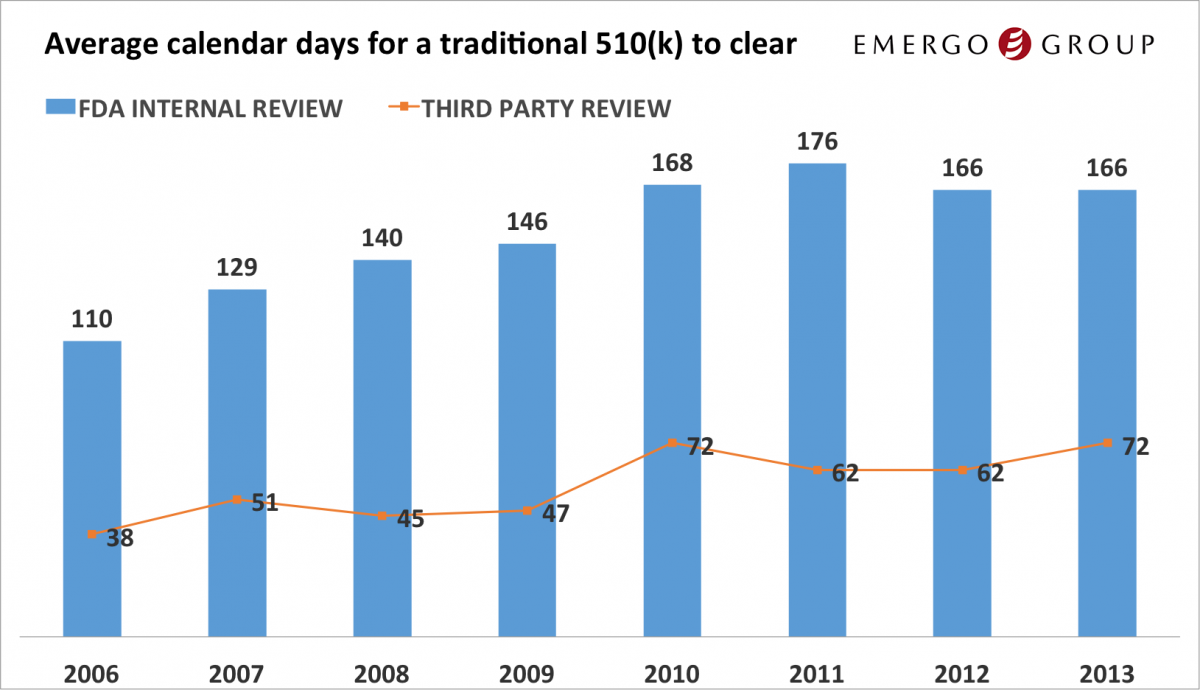 On average it took 166 days (nearly 5 months) for the FDA to review and clear a 510(k) in 2013
On average it took 166 days (nearly 5 months) for the FDA to review and clear a 510(k) in 2013- Third Party Reviewers review 510(k) submissions in an average of 72 days
- The amount of time it takes the FDA to review a 510(k) has stabilized
- Radiology products clear faster than average; dental products take more time
- RTA policy impact on review times still unclear
CLICK HERE FOR OUR FULL ANALYSIS including even more interesting charts like the one shown, and see trending from 2006 through 2013.