Get smart about Brazil in 1 hour
有任何问题?向我们的专家获取相关信息
联系我们If you are thinking about entering the Brazilian market for the first time, understanding ANVISA’s regulatory requirements can be overwhelming. Information about medical device regulations on the ANVISA website can be confusing and does not tell the full story about how the registration process really works, or what is required of you as a manufacturer.
Free information packet makes it easier to understand the process in Brazil
To help medical device manufacturers who want to export medical devices to Brazil, we assembled a packet that includes the basic information you need before deciding to enter the Brazilian market. It outlines the registration requirements and ongoing obligations to maintain compliance.
Here is what we will send you
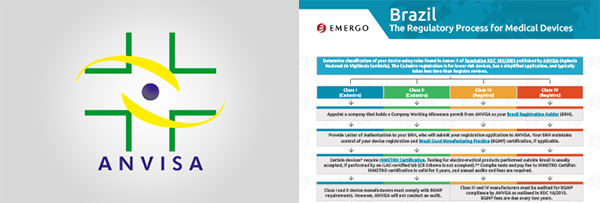

If you set aside just one hour to read/watch these items, we guarantee you will have a MUCH better understanding of how ANVISA’s regulatory process works.
EMAIL INFORMATION PACKET TO ME
No salesperson will call you
相关
-
美国FDA eSTAR电子递交模板与资源要求解析
自2018年起,美国食品和药物管理局(U.S. Food and Drug Administration,FDA)开始试行以电子化方式来帮助行业提供完整的510(k)上市前通知。2022年,为推进以电子格式提供510(k)递交文件的过渡,美国FDA正式发布关于使用电子递交模板和资源的最终指导文
阅读更多 -
针对体外诊断医疗器械的欧盟IVDR符合性评定选项
自2022年5月26日始,欲在欧盟(EU)上市的新型体外诊断(IVD)医疗器械必须符合欧盟体外诊断医疗器械法规(2017/746 IVDR)。 同日起,带有有效CE标志的IVD可以继续按照指令98/79/EC(IVDD)进行销售,直至其许可证到期为止。 自2025年5月27日始,在欧盟销售的所
阅读更多